Tim Sowers , Don Vanderlaan, Andrei Karpiouk, Eleanor Barber, Ph.D., Ethan Smith
Summary
Intravascular photoacoustic imaging is capable of identifying atherosclerotic plaques but requires large cumulative dosages of light to image the vessel wall. Currently, no guidelines exist for the maximum safe light dosage that can be applied to tissue. Higher dosages are desirable because they provide better image quality, although damage must be avoided. We’ve conducted in vitro studies on cells that indicate dosages will need to be limited below that used in some studies in the literature, although there is plenty of scope to lower the dosage to safe levels while maintaining adequate imaging quality for plaque identification. Future work on this project includes in vivo studies on healthy and atherosclerotic swine.
Description
Intravascular photoacoustic imaging of arteries requires significantly more photoacoustic acquisitions than most biomedical imaging applications. This is because the catheter, which is only large enough to hold one ultrasound transducer, is side firing. As a result, it must be rotated while acquiring multiple photoacoustic acquisitions. The 2D cross section of the vessel is then constructed by combining about 100 of these acquisitions. Creating 3D images is done by simultaneously rotating and pulling back the catheter. The result is that imaging a stretch of tissue several centimeters long requires thousands of laser pulses. From this, concerns about damage to the vessel wall tissue have arisen. Although safety standards exist for the application of laser light to the eye and skin, no such standards exist for the application of laser light to arteries leaving researchers without clear guidance.
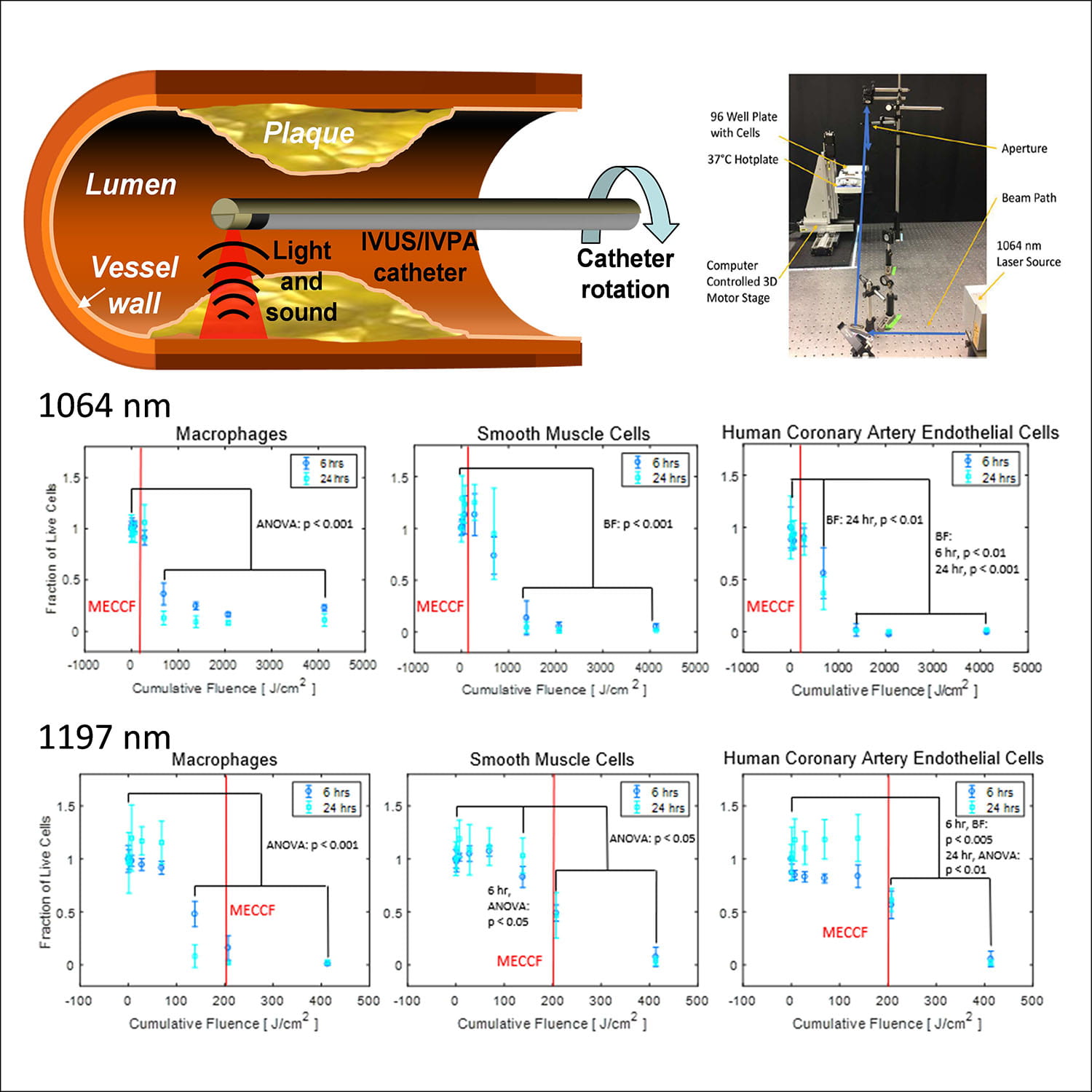 We are investigating the safety of intravascular photoacoustic imaging in vitro, ex vivo, and in vivo. Our first results have been in vitro. We irradiated several cell types commonly found in healthy and diseased blood vessels – endothelial cells, macrophages, and smooth muscle cells. We subjected these cells to various amounts of cumulative irradiation over many pulses (fluences [units: energy/area]) at 1064 nm and 1197 nm. Lipid absorbs strongly at the latter wavelength while 1064 nm could be easily used for dual wavelength imaging due to the wide use of Nd:YAG lasers. The cells were evaluated for viability 6 and 24 hours after irradiation. The fluence at which death occurred was compared to the maximum expected clinical cumulative fluence (MECCF) during a course of clinical imaging, which was estimated using conservative (meaning higher fluence) intravascular photoacoustic imaging protocols found in the literature. We found that cell death does occur at the highest levels of expected clinical cumulative fluence, although imaging could be completed at much lower cumulative fluences. We also determined that damage is likely to be the result of tissue heating, rather than optical damage from high fluences of single laser pulses. Ultimately, higher dosages will result in improved image quality, while lower dosages will reduce the likelihood of damage. The goal of our research is to find safe imaging protocols that allow for the highest possible imaging quality.
We are investigating the safety of intravascular photoacoustic imaging in vitro, ex vivo, and in vivo. Our first results have been in vitro. We irradiated several cell types commonly found in healthy and diseased blood vessels – endothelial cells, macrophages, and smooth muscle cells. We subjected these cells to various amounts of cumulative irradiation over many pulses (fluences [units: energy/area]) at 1064 nm and 1197 nm. Lipid absorbs strongly at the latter wavelength while 1064 nm could be easily used for dual wavelength imaging due to the wide use of Nd:YAG lasers. The cells were evaluated for viability 6 and 24 hours after irradiation. The fluence at which death occurred was compared to the maximum expected clinical cumulative fluence (MECCF) during a course of clinical imaging, which was estimated using conservative (meaning higher fluence) intravascular photoacoustic imaging protocols found in the literature. We found that cell death does occur at the highest levels of expected clinical cumulative fluence, although imaging could be completed at much lower cumulative fluences. We also determined that damage is likely to be the result of tissue heating, rather than optical damage from high fluences of single laser pulses. Ultimately, higher dosages will result in improved image quality, while lower dosages will reduce the likelihood of damage. The goal of our research is to find safe imaging protocols that allow for the highest possible imaging quality.
Our future work will include in vivo studies in the arteries of swine. Multiple dosages of light will be applied to the artery, and the arteries will be evaluated for damage from heat. In this study, we will use 1064 nm light and 1720 nm light, which corresponds to an absorption peak of lipid but also has a higher absorption peak than light near 1200 nm. Thus, we expect light at 1720 nm will result in a lower damage threshold. Planned studies will include irradiation of arteries in both healthy and atherosclerotic swine.
Further Reading
- Sowers et al. Laser threshold and cell damage mechanism for intravascular photoacoustic imaging. Lasers Surg Med. (2018) 51(5) doi: https://doi.org/10.1002/lsm.23026
- Sowers et al. Determining the laser damage threshold of tissues for the design of a clinical IVPA imaging protocol. IEEE International Ultrasonics Symposium (IUS). Sept 2017. Washington, D.C. doi: 10.1109/ULTSYM.2017.8092569
References
- Bo Wang and Stanislav Emelianov. Thermal intravascular photoacoustic imaging. Biomedical Optics Express, vol 2, issue 11, pp. 3073-3078 (2011). doi: 10.1364/BOE.2.003072
