Tim Sowers , Nich Dana, Heechul Yoon, Don Vanderlaan, Andrei Karpiouk
Summary
Increasing photoacoustic signal from an imaging target promises enhanced diagnostic accuracy. Here, we use Monte Carlo photon propagation simulations to optimize diagnostic accuracy in a variety of photoacoustic applications. Fundamentally, the strength of a photoacoustic signal is proportional to the amount of light (measured in fluence [units: energy/area]) that is delivered to the area of the imaging target, the optical absorption coefficient of the imaging target, and physical properties of the imaging target that are lumped together and referred to as the Grunesein coefficient. Using Monte Carlo modeling of photon propagation, it is possible to determine the amount of light delivered to a specific region of tissue. Since the tissue types will be defined in this model, the optical absorption coefficients and Gruneisen coefficients will also be known in each location. This information can be used to map the intensity of photoacoustic signal in a simulated volume at different wavelengths, laser energies, or experimental setups. In our lab, we first used this method to compare the two optimal wavelengths for identifying atherosclerotic plaques. More recently, we’ve also used this to determine the optimal experimental geometry for light delivery, with a focus on determining differences between mice and humans.
Description
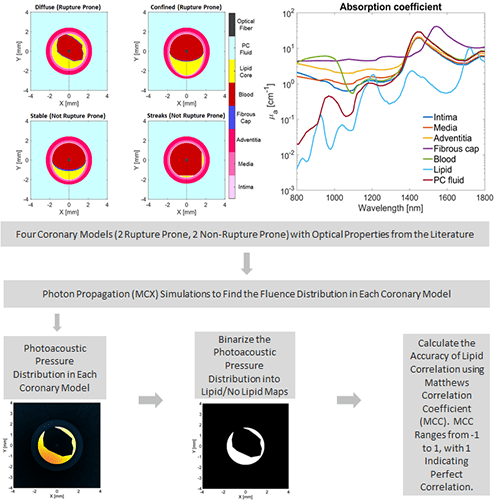
Our first project investigated the best two wavelengths to use for identification of atherosclerotic plaques. Generally, the strength of a photoacoustic signal from a target is proportional to the optical absorption of the target at the wavelength of light used for irradiation. If the optical absorption of a target is known across a variety of wavelengths, then it can be identified by acquiring photoacoustic images over a range of wavelengths and determining if the variation in photoacoustic intensity over the wavelengths matches what would be expected based on the known optical absorption properties. This is referred to as spectroscopic imaging. However, intravascular photoacoustic imaging requires thousands of photoacoustic acquisitions (and hence thousands of laser pulses) to construct a single 3D image. This makes it unfeasible to image using a variety of wavelengths, since the acquisition time would become too long.
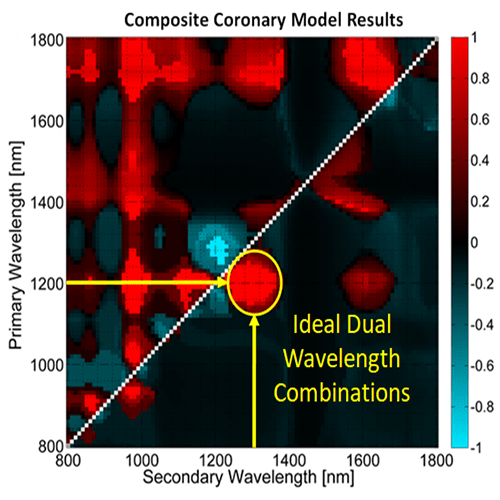
However, imaging with two wavelengths would minimize the increase in acquisition time while still providing some of the benefit of multiwavelength imaging. We have conducted research to determine the optimal two wavelength combination that could be used. To achieve this, we used Monte Carlo modeling to simulate the light propagation and absorption through blood and vessel tissue. Light propagation was simulated in four vessel models (2 healthy, 2 with significant plaques). These models were constructed using optical properties of tissue types in the vessel wall from the literature. The propagation of light was used to determine the photoacoustic pressure distribution in each coronary model for wavelengths ranging from 800-1800 nm. The pressure distribution was then used to calculate the locations at which lipid was identified with the simulation for every two wavelength combination. These were then compared to the known coronary artery models to determine the relative accuracy of each dual wavelength combination. We found that using a primary wavelength at 1210 nm and a secondary wavelength near 1300 nm resulted in the most accurate lipid detection.
In the second project, we simulated the optimal geometry for a photoacoustic imaging setup consisting of a linear ultrasound transducer array and light delivery from the side using an optical fiber. This is a commonly used imaging modality, since the system takes advantage of linear ultrasound transducer array technology which has been heavily developed for clinical applications. We investigated the effect of different angles of incidence for light delivery, and changing distance between the location of light delivery and the ultrasound transducer. We focus on the differences in optimal geometry arising between mice and humans. Our findings have important implications for the translation of imaging methods developed in mice models in the lab to humans in the clinic. This work is currently under review.
Further Reading
- Dana et al. Optimization of dual-wavelength intravascular photoacoustic imaging of atherosclerotic plaques using Monte Carlo optical modeling. Journal of Biomedical Optics. (2017) 22(10). doi: 10.1117/1.JBO.22.10.106012
- Sowers et al. Optimization of dual wavelength IVPA imaging for accurate detection of lipid in atherosclerotic plaques. IEEE International Ultrasonics Symposium (IUS). Sept 2017. Washington, D.C. doi: 10.1109/ULTSYM.2017.8092700