Summary
Intravascular photoacoustic imaging is a relatively new modality which takes advantage of the unique optical absorption spectra of lipid to locate atherosclerotic plaques, a large and growing cause of mortality worldwide. However, contrast from lipid alone is limited and does not provide information about the likelihood that a specific plaque will be a root cause of mortality. Nanoscale contrast agents are used by our lab to address both these shortcomings. The small size of these agents result in their diffusion into atherosclerotic plaques, due to the permeability of the endothelial layer found over atherosclerotic regions of tissue. The nanoscale agents have high absorption coefficients, resulting in large photoacoustic signals that are easily identifiable. In addition, they can be conjugated with antibodies that make them adhere to specific biological markers, making it possible to use the presence of these agents as an indicator of plaque growth or rupture.
In intravascular photoacoustic imaging, the high optical absorption of blood in the vessel lumen reduces the amount of light that travels from its exit at the catheter tip to the vessel wall. The strength of a photoacoustic signal is proportional to the amount of light (specifically, fluence [units: energy/area]) that reaches the target. As a result, getting strong signals in intravascular photoacoustic imaging can be difficult, particularly at the longer wavelengths (1210 & 1720 nm) that are used to image endogenous lipid. To enhance the photoacoustic signal and improve plaque detection and characterization, our lab uses contrast agents. These agents are nanoscale particles that have strong absorption and will collect in atherosclerotic plaques.
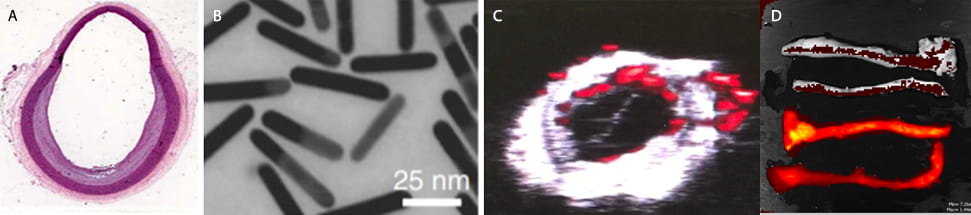
The agents our lab use include metallic nanoparticles, dyes, and more complex particles consisting of a phospholipid shell which may or may not be loaded with other strongly absorbing particles on the interior. In addition, many of these agents can be conjugated with antibodies that selectively bind to specific molecules. Molecules are chosen that are present in higher concentrations in atherosclerotic plaques, particularly those plaques that are prone to rupture or erosion, which in turn cause the heart attacks and strokes associated with atherosclerotic disease. Thus, targeting could give a way for clinicians to both identify plaque and determine patient specific risk.
Past work in the lab involved using gold nanorods (< 100 nm), which has strong absorption properties. These particles naturally diffuse into plaque and are taken up by macrophages, a cell which in high concentrations is an indicator of rupture likelihood. However, the absorption of these specific gold particles was not ideal for intravascular photoacoutics imaging, since it is difficult to obtain high power lasers at the absorbing wavelength of these gold rods. Because of these, we’ve begun studies using recently discovered methods for synthesizing gold particles that have an optical absorption peak near 1064 nm, the wavelength used for the commonly used Nd:YAG laser. This would make real time imaging of these rods possible in the clinic.
While we use these agents mainly to improve imaging, future work can also explore how the particles could be used for therapy. Irradiation of gold nanoparticles will cause them to heat both themselves and surrounding tissue, potentially destroying cells (e.g. macrophages) in the plaque that are involved in the biological processes leading to rupture and erosion.
Further Reading
- Sowers, T. and Emelianov, S. Exogenous imaging contrast and therapeutic agents for intravascular photoacoustic imaging and image-guided therapy. Phys Med Biol. (2018) 63(22) doi: 10.1088/1361-6560/aae62b
References
- Yeager et al. Intravascular photoacoustic imaging of exogenously labeled atherosclerotic plaque through luminal blood. J Biomed Opt. (2012) 17(10): 106016 doi: 10.117/1.JBO.17.10.106016
- Chen, Yun Sheng et al. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nature Nanotechnology. (2019) 14: 465-472 doi: https://doi.org/10.1038/ s41565-019-0392-3
