Donald VanderLaan , Andrei Karpiouk , Tim Sowers , Muralidhar Padala, PhD
Summary
Intravascular photoacoustic (IVPA) imaging is a promising new imaging modality with significant applicability to cardiovascular disease, in particular, assessment of vulnerable plaques. Photoacoustic imaging offers molecular and functional information not available via other catheter-based imaging technologies. IVPA pairs excellently with intravascular ultrasound (IVUS) imaging since they share a substantial amount of hardware while offering complimentary information (IVUS yields anatomy/morphology). Though not clinically deployed, IVPA has demonstrated superior abilities to identify plaque composition via optical excitation in spectral regions where lipid absorbs more strongly than water-based tissues.
Description
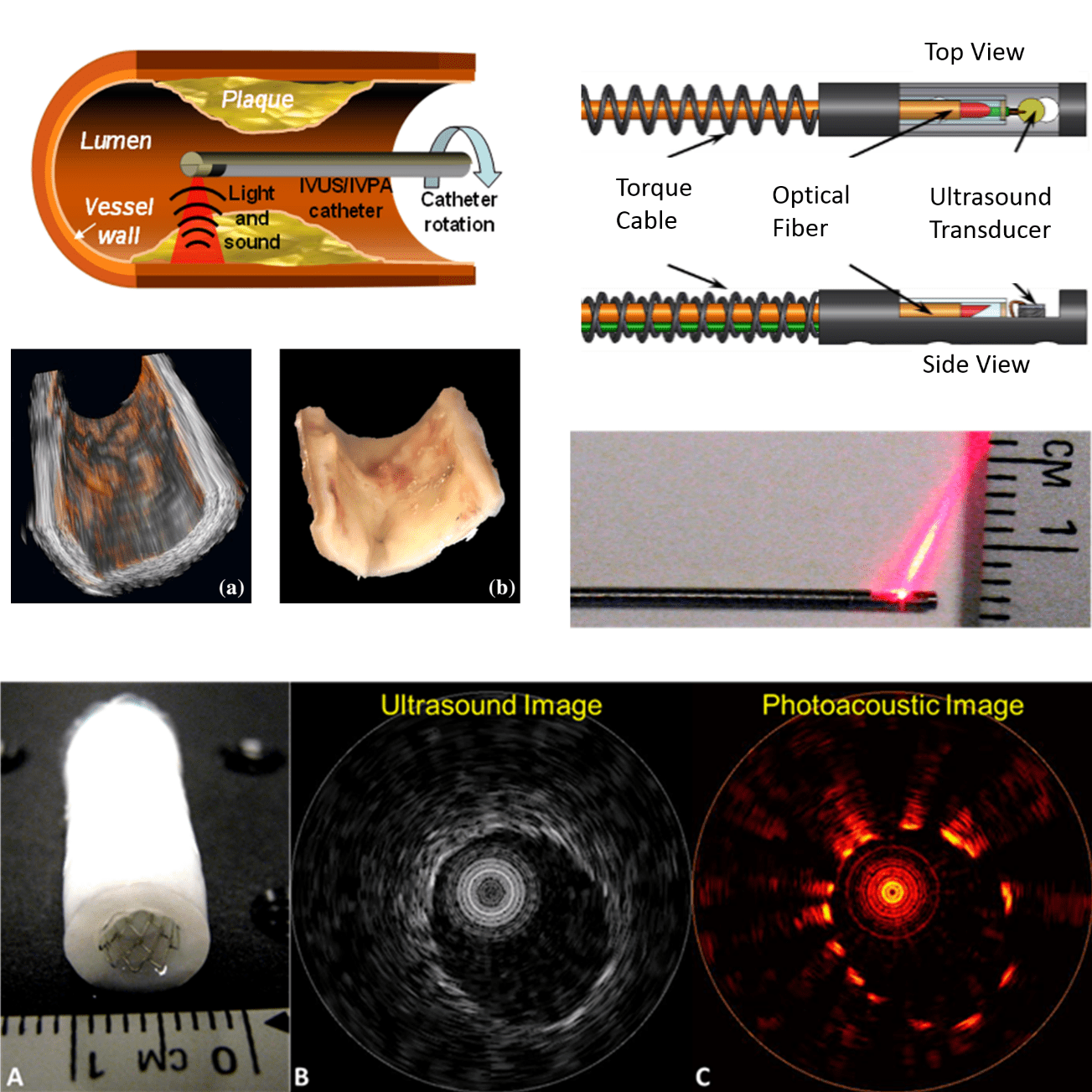 Intravascular photoacoustic (IVPA) imaging is a promising new imaging modality with an extensive record of encouraging results in preclinical research. Photoacoustic imaging offers functional and compositional information similar to MRI or PET/SPECT, while simultaneously being cheap and providing results in real time. IVPA is the catheter-based implementation of photoacoustic imaging, permitting imaging deep within the chest which would not be possible with externally-deployed photoacoustic imaging. IVPA is ideally paired with intravascular ultrasound (IVUS), which itself is already extensively used clinically. While IVUS provides anatomical imaging, IVPA compliments with compositional and functional imaging.
Intravascular photoacoustic (IVPA) imaging is a promising new imaging modality with an extensive record of encouraging results in preclinical research. Photoacoustic imaging offers functional and compositional information similar to MRI or PET/SPECT, while simultaneously being cheap and providing results in real time. IVPA is the catheter-based implementation of photoacoustic imaging, permitting imaging deep within the chest which would not be possible with externally-deployed photoacoustic imaging. IVPA is ideally paired with intravascular ultrasound (IVUS), which itself is already extensively used clinically. While IVUS provides anatomical imaging, IVPA compliments with compositional and functional imaging.
The primary focus of IVPA research is cardiovascular disease, one of the leading causes of premature death worldwide. A particular facet concerns arterial plaque distribution and composition. Lipid is readily distinguished from other arterial tissue constituents by its unique optical absorption spectrum – the fundamental physical property exploited by IVPA to quantify tissue composition. The information obtained – lipid type, volume, etc – aids in the determination of how hazardous it is and could guide treatment strategy.
The absorption spectrum of plaques of interest is dominated by peaks near 915, 1210, and 1720nm. Near these regions, the absorption coefficient is several-fold higher than water (which dominates the absorption spectrum of other tissues in the NIR). Using absorption spectroscopy – or even simply single wavelength imaging at one of the peaks – plaques are distinguished and can be morphologically characterized. Through the use of cell-targeted nano-particle contrast agents, macrophage density within the plaque region can also be studied. Detecting vulnerable (rupture-prone) plaque can guide treatment and avert serious consequences down the road.
Another direction we are investigating is the combination of IVPA and intravascular optical coherence tomography (IVOCT). IVOCT is a competitor to IVUS and is slowly gaining clinical acceptance, despite not being covered by most insurances. The combination of IVOCT and IVPA could be of paramount utility for assessing and guiding treatment of one particular type of vulnerable plaque: thin-cap fibroatheroma (TCFA). TCFA manifests as a voluminous necrotic lipid core containing numerous macrophages, and covered with a thin (<65 micron) fibrous cap. TCFA is dangerous because of the likelihood of rupture, often the precursor to the majority of coronary thrombi. The IVOCT / IVPA combination is unique in that IVOCT is capable of easily assessing the thickness of the fibrous cap, while IVPA informs plaque content. With the addition of cell-specific contrast agents, IVPA further offers the ability to gauge macrophage density. These three features comprise the defining aspects of TCFA.
Our group pioneered IVPA a decade ago, and our major contributions span catheter design, the first real-time IVPA system, first live animal stent imaging, and IVPA contrast agents / nanoparticles. Our future work will include in vivo studies in the arteries of swine. Several aspects this new study will cover are: 1) Safety of IVPA, and optimal optical parameters for imaging while maintaining safety, 2) Real-time multi-wavelength semi-spectroscopic IVPA imaging, 3) Plaque identification in a swine model genetically-bred for cardiovascular disease, 4) Further development of our catheter device, including ability to navigate from femoral access all the way to the coronary arteries, among several other objectives.
Further Reading
- T Sowers, D VanderLaan, A Karpiouk, E Donnelly, E Smith, S Emelianov. Laser threshold and cell damage mechanism for intravascular photoacoustic imaging. Lasers in Surgery and Medicine. (2018) 51(5):466-74
- D VanderLaan, A Karpiouk, D Yeager, S Emelianov. Real-time intravascular ultrasound and photoacoustic imaging. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. (2017) 64(1):141-149
- A Karpiouk, B Wang, J Amirian, R Smalling, S Emelianov. Feasibility of in-vivo intravascular photoacoustic imaging using integrated ultrasound and photoacoustic imaging catheter. J Biomedical Optics. (2012) 17(9)
- Sowers, T. and Emelianov, S. Exogenous imaging contrast and therapeutic agents for intravascular photoacoustic imaging and image-guided therapy. Phys Med Biol. (2018) 63(22) doi: 10.1088/1361-6560/aae62b
- D Yeager, Y-S Chen, S Litovsky, S Emelianov. Intravascular photoacoustics for image-guidance and temperature monitoring during plasmonic photothermal therapy of atherosclerotic plaques: a feasibility study. Theranostics. (2014) 4(1):36-46
- N Dana, T Sowers, A Karpiouk, D VanderLaan, and S Emelianov. Optimization of dual-wavelength intravascular photoacoustic imaging of atherosclerotic plaques using Monte Carlo optical modeling. J Biomedical Optics. (2017) 22(10)
- A Karpiouk, B Wang, and S Emelianov. Development of a catheter for combined intravascular ultrasound and photoacoustic imaging. Review of Scientific Instruments. (2010) 81:1
- B Wang, A Karpiouk, D Yeager, J Amirian, S Litovsky, R Smalling, and S Emelianov. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood. Optics Letters. (2012) 37(7):1244-6
- B Wang, E Yantsen, T Larson, A Karpiouk, S Sethuraman, J Su, K Sokolov, and S Emelianov. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Letters. (2009) 9(6):2212-17
- B Wang, J Su, J Amirian, S Litovsky, R Smalling, and S Emelianov. Detection of lipid in atherosclerotic vessels using ultrasound-guided spectroscopic intravascular photoacoustic imaging. Optics Express. (2010) 18(5):4889-97
- S Sethuraman, S Aglyamov, J Amirian, R Smalling, S Emelianov. Intravascular photoacoustic imaging using an IVUS imaging catheter. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. (2007) 54(5):978-86
References
- Virmani R, Burke AP, Kolodgie FD, Farb A. Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. Journal of Interventional Cardiology. (2003) Jun; 16(3):267-72
